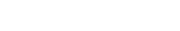Can rapid tooling truly achieve the micron-level precision required for medical device certification? Yes. By combining advanced mold flow simulation, hybrid additive manufacturing, and rigorous process validation, rapid tooling has evolved from a simple prototyping method into a compliant bridge production solution. This guide explains how to leverage these technologies to meet FDA and ISO 13485 standards, ensuring your Class I and II devices pass clinical evaluation and reach the market faster without compromising on safety or tolerance.
Navigating Regulatory Compliance via Rapid Manufacturing
To successfully deploy rapid tooling in a regulated environment, engineers must align manufacturing lifecycles with FDA classification pathways, ensuring that early-stage bridge tools provide data applicable to final validation.
Aligning Tooling Lifecycles with Certification Paths
For Class I and II devices, the distinction between a non-clinical prototype and a clinical trial unit is critical. Rapid tooling bridges this gap by producing parts in end-use materials (such as USP Class VI PEEK or medical-grade Polycarbonate) rather than surrogate 3D printed resins. This allows Quality Assurance teams to perform functional testing on units that are mechanically identical to mass-production parts. Utilizing rapid application tools for DFM (Design for Manufacturing) analysis early in the design phase helps identify potential compliance risks—such as non-fill areas or air traps—before steel is cut. This proactive approach prevents costly revalidation cycles often triggered by major tooling modifications during the New Product Introduction (NPI) phase.
Validating Process Parameters for ISO 13485 Audits
Regulatory bodies require more than just a correct part; they require a validated process. When using rapid molds for bridge production, it is essential to establish scientific molding parameters (Injection Pressure, Hold Time, Cooling Rate) that demonstrate repeatability. Documentation of Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) on rapid aluminum molds provides the necessary traceability for ISO 13485 audits. This data serves as a benchmark when transitioning to high-volume multi-cavity steel molds, significantly reducing the “time-to-confidence” for final regulatory approval.
Achieving Micron-Level Precision in Mold Fabrication
Modern rapid tooling strategies now rival traditional machining in accuracy, utilizing hybrid manufacturing techniques to achieve tolerances as tight as ±0.005 mm for critical features in medical assemblies.
Utilizing Hybrid Tooling for Complex Geometries
Achieving high fidelity in micro-features—such as fluid channels in diagnostic chips or texture on surgical grips—requires a hybrid approach. Advanced rapid tooling suppliers often combine standard MUD (Master Unit Die) bases with conformal-cooled inserts created via Direct Metal Laser Sintering (DMLS). This allows for complex cooling channels that traditional drilling cannot create, ensuring uniform thermal dissipation. Uniform cooling is vital for minimizing warpage in thin-walled medical parts, thereby maintaining dimensional stability across thousands of shots. This method supports the creation of complex geometries without the long lead times associated with EDM (Electrical Discharge Machining) of hardened steel.
Executing Scientific Molding for Repeatability
Precision is not just about the mold; it is about the injection process. Scientific molding principles enable molders to decouple the filling and packing stages, ensuring that variations in resin viscosity do not affect the final part dimensions. By using cavity pressure transducers within the rapid tool, manufacturers can monitor the exact conditions inside the mold in real time. This level of process control ensures that every bridge part meets the same strict acceptance criteria as full production units, allowing for immediate deployment in clinical trials or market entry while high-cavity tools are being built.
Livepoint Tooling: Precision Manufacturing Solutions
Livepoint Tooling specializes in accelerating product development through high-precision manufacturing services designed for demanding industries like medical devices and automotive systems.
Key Service Capabilities:
Rapid Tooling & Injection Molding: Livepoint offers fast-turnaround mold fabrication, allowing clients to bridge the gap between prototyping and mass production. Their expertise in processing engineering-grade thermoplastics ensures parts meet functional requirements for Class I/II medical applications.
CNC Machining: Utilizing advanced multi-axis CNC centers, Livepoint delivers tight-tolerance metal and plastic components. This service is essential for creating durable fixtures, jigs, and low-volume metal enclosures that require superior surface finishes.
Sheet Metal Fabrication: From brackets to complex chassis, their sheet metal services support the creation of structural components necessary for industrial and medical equipment housings.
Conclusion:
By integrating speed with strict quality management systems, Livepoint Tooling enables engineers to reduce time-to-market without sacrificing precision. Their comprehensive suite of manufacturing options allows for seamless scaling from initial design to final production.
Partner with Livepoint Tooling today to secure high-precision rapid tooling that accelerates your certification timeline and ensures regulatory compliance.